In many hemato-oncology and pathology laboratories, IG/TR clonality testing is part of the daily diagnostic work-up for samples with suspected lymphoproliferations or lymphoma. Standardization of PCR protocols for the detection of clonal proliferations in lymphoproliferative disease was established by the BIOMED-2/EuroClonality Consortium (JJM van Dongen et al, Leukemia 2003:17:2257-2317, for literature and protocols see EuroClonality website,FAQs ). These protocols are now well established in many labs, partly due to their commercial availability. These kits have made the technical performance of these protocols relatively uncomplicated. However, the technical interpretation is less straightforward, since the reporting of these assays requires knowledge of T-cell receptor (TR) and immunoglobulin (IG) gene rearrangements, multiple rearrangements patterns and cross-lineage rearrangements.
). These protocols are now well established in many labs, partly due to their commercial availability. These kits have made the technical performance of these protocols relatively uncomplicated. However, the technical interpretation is less straightforward, since the reporting of these assays requires knowledge of T-cell receptor (TR) and immunoglobulin (IG) gene rearrangements, multiple rearrangements patterns and cross-lineage rearrangements.
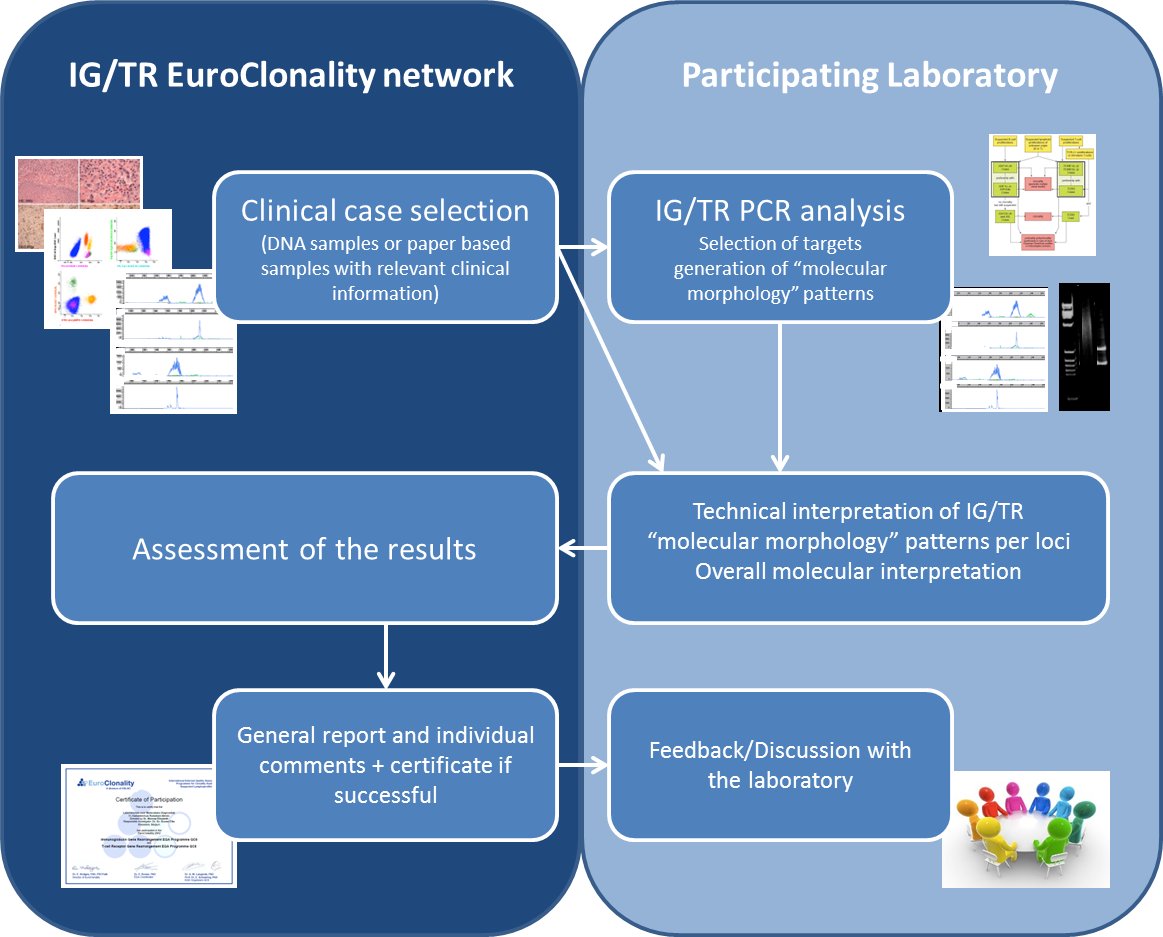
To improve the standardization and reporting of clonality analysis, the EuroClonality Consortium, which comprises more than 20 laboratories across Europe with considerable technical experience with the BIOMED-2/EuroClonality primers and protocols, started a series of quality assessment (QA) schemes in 2008.
Two different kinds of QA rounds were organized: paper trials and trials with DNA extracted from different hematological and histological cases, which participants analyzed according to their routine diagnostic PCR practice (GeneScan and/or heteroduplex analysis) based on the suspected diagnosis. Paper trials focused on the interpretation of GeneScan and/or heteroduplex results, whereas the DNA trials assessed both the correct generation of PCR results as well as their interpretation. Participants were asked to submit results for individual loci tested, give an overall molecular interpretation and a final clinical interpretation where appropriate. These schemes resulted in the published guidelines for the analysis, interpretation and reporting of the BIOMED-2/EuroClonality assays (Langerak et al., Leukemia 2012;26:2159-2172, see FAQs EuroClonality website). To further standardize clonality reporting, language specific translations of these guidelines are available at the EuroClonality website. However, as these guidelines start from a immunobiological concept, it should be stressed that they can be applied to any clonality result whether or not generated with BIOMED-2/EuroClonality protocols.
EuroClonality website). To further standardize clonality reporting, language specific translations of these guidelines are available at the EuroClonality website. However, as these guidelines start from a immunobiological concept, it should be stressed that they can be applied to any clonality result whether or not generated with BIOMED-2/EuroClonality protocols.
Likewise for this scheme, both paper based and DNA based cases will be distributed annually. Participants need to test and/or interpret the EQA samples according to their laboratory’s routine practice, with the aim to improve and standardize diagnostic practices.
The practical organization of this European EQA program is done in collaboration with Dr Bart Lubbers (ESLHO), Dr Elke Boone QA lead for EuroClonality, Dr Paula Gameiro, and the Biomedical Quality Assurance Research Unit of the University of Leuven led by Prof Dr Els Dequeker. The scheme organizers are participants of the EuroClonality Consortium and will be in close contact with the European QA program coordinator. The EQA schemes are accredited by BELAC conform the ISO 17043
 , (PT-215), which is the international standard for conformity assessment of proficiency testing.
, (PT-215), which is the international standard for conformity assessment of proficiency testing.
In this EQA scheme, the tasks of preparing and sending the samples are assigned to laboratories within the EuroClonality network, who are in close contact with the EuroClonality QC lead. Together with the EuroClonality Board, these parties have the final responsibility for the EQA scheme.
Samples are presented as clinical cases and relevant clinical, flow cytometry and/or immunostaining data will be provided. As both hemato-oncology and pathology labs perform clonality analysis, sometimes for different clinical investigations, typical sample types (e.g. peripheral blood or FFPE tissue) will be included on a regular basis.
Five IG cases and five TR cases will be distributed together annually. Each trial round will be composed of 5 paper based cases and 5 DNA samples extracted from a variety of different lymphoproliferations. A rotation system will be applied so that after 2 years 5 paper based and 5 DNA samples for the IG scheme will be sent around. A similar rotation system will be applied for the TR scheme.
This year, participants will receive the following cases:
The materials distributed are provided as specimens for the sole purpose of enabling external quality assessment at the recipient’s laboratory during the current distribution and must not be tested for any other target than that which is requested by the EQA scheme. They do not contribute in vitro medical diagnostic devices (IVDs), and no claim is made that they may be suitable for any other purpose or at any other point in time.
The scheme organizers prepare the samples and distribute them to the participants. Cases are pretested by at least 3 laboratories of the EuroClonality Consortium to determine the correct result. Coordination and evaluation of the results is done by the EuroClonality EQA scheme coordination center, as well as the EuroClonality QC lead.
Laboratories can register either for the IG trial, the TR trial or both. A fee of 420€ per gene target (IG or TR) or 840€ for both will be asked to the participants for the organization cost, preparation and sending of the samples and assessment of the results.
After registration, each participant will receive a username and password that will allow them to log in to the EQA participants area where both sample information and links for result submission can be found.
After closure of registration, participants will be notified by email of their participation and will receive an invoice from EuroClonality.
Each year, all laboratories that have an account will receive an invitation via email to register online for the subsequent schemes. An identification number, the EQA ID number, is assigned to each participant automatically upon registration.
Registrations for the 2020 scheme were run on the new website . Results submission will be done on this new platform as well.
. Results submission will be done on this new platform as well.
The coordination center in Leuven will coordinate the evaluation of the submitted results. Results of the EQA schemes will be announced after discussion within the EuroClonality Consortium. These results will be made available anonymously among the participants but each participating laboratory will receive individual feedback.
Scoring will initially be done on the final “Molecular Interpretation/Conclusion” of each case and not on the interpretation of the individual PCR results, allowing laboratories who use non-BIOMED-2/EuroClonality protocols also to participate. However, in the final report results of BIOMED-2/EuroClonality PCR targets will be discussed in detail and feedback will be given.
The correct “Molecular Interpretation/Conclusion” was determined by at least 3 EuroClonality laboratories during a pretesting phase. However, a case might be withdrawn from the EQA scoring in case high discordant results are obtained by the participants.
The IG/TR clonality EQA Scheme allows participants to react on the score and/or comments they received. All appeals should be sent via email to before the deadline for appeals that is indicated in the general report. The appeals will be collected and will be discussed by the assessors. The laboratories will receive an individual answer and after this, the marks become final.
Laboratories who are requested to submit a corrective action plan are required to do so within the timeframe stipulated in the general report. The corrective actions forms can be downloaded after login in the EQA participants area.
All participants receive a certificate of participation and an individual performance letter after the appeal phase.
Laboratories will receive a certificate of good performance for either IG or TR analysis or both, after evaluation of 10 samples. After two participations, at least 9 out of 10 cases should have been correctly assessed as determined by the “European Consensus Conference for External Quality Assessment in Molecular Pathology” (van Krieken, et al., Virchows Arch 2013;462:27-37).
(van Krieken, et al., Virchows Arch 2013;462:27-37).
Laboratories that participated successfully, will be listed on the EuroClonality website after the appeal phase if consent was given during the registration.












The fact of participation, the raw data and the individual report are confidential information between the individual laboratory and the scheme organizer as well as the European QA coordinator.